Acquired Immune System
B cell
T helper and T cytotoxic
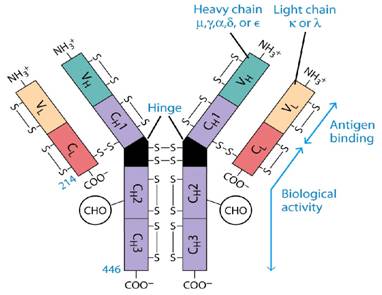
B Cells

2 light and two heavy chains
Disulfide bonded (intra and inter)
5 Classes of Ig's
IgG
IgA
IgE
IgM
IgD
Antigenic Determinants (Epitopes)

Low Response
IgM response.
T cell independent
High Response
T cell dependent
IgG now becomes involved
Q. What is the biggest benefit of an IgG response?
Sequence of Events
Ag recognized by Ab on B cell
Ingested by B cell and immunogen broken down
Select peptides of immunogen presented on surface of B cell via MHC (B cell is now acting as an antigen presenting cell. APC)
Peptide is presented to T helper cell via TCR.
Th cells (CD4)
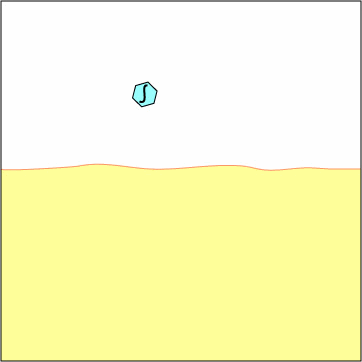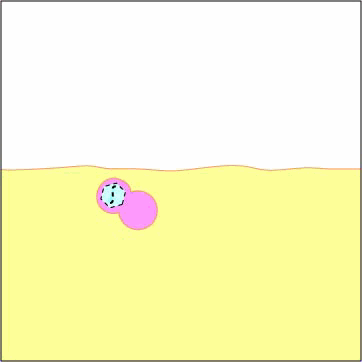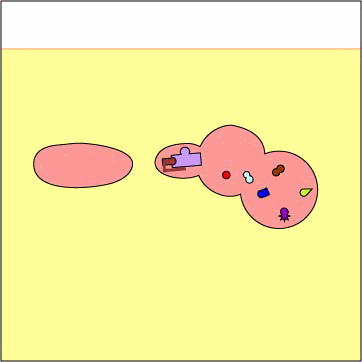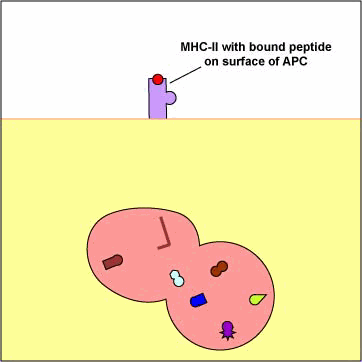
Exogenous antigens are those from outside cells of the body. Examples include bacteria, free viruses, yeasts, protozoa, and toxins. These exogenous antigens enter antigen-presenting cells or APCs (macrophages, dendritic cells, and B-lymphocytes) through phagocytosis. The microbes are engulfed and placed in a phagosome. After lysosomes fuse with the phagosome, protein antigens are degraded by proteases into a series of peptides. These peptides eventually bind to grooves in MHC-II milecules and are transported to the surface of the APC. T4-lymphocytes are then able to recognize peptide/MHC-II complexes by means of their T-cell receptors (TCRs) and CD4 molecules.
1. Exogenous antigens, such as viruses,
are engulfed and placed in a phagosome.
2. Lysosomes fuse with the phagosome forming an phagolysosome.
3. Protein antigens are degraded into a series of peptides.
4. MHC-II molecules are synthesized
in the endoplasmic reticulum and transported to the Golgi complex. Once assembled,
wihtin the endoplasmic reticulum, a protein called the invarient chain (Ii)
attaches to the the peptide-binding groove of the MHC-II molecules and in this
way prevents peptides designated for binding to MHC-I molecules within the ER
from attaching to the MHC-II.
5. The MHC-II molecules with bound Ii chain are now transported to the Golgi
complex, and placed in vesicles.
6&7. The vesicles containing the MHC-II molecules fuse with the peptide-containing
phaglysosomes. The Ii chain
is removed and the peptides are now free to bind to the grooves of the MHC-II
molecules.
8. The MHC-II molecules with bound peptides are transported to the cytoplasmic
membrane where they become anchored. Here, the peptide and MHC-II complexes
can be recognized by T4-lymphocytes by way of TCRs and CD4 molecules having
a complementary shape.
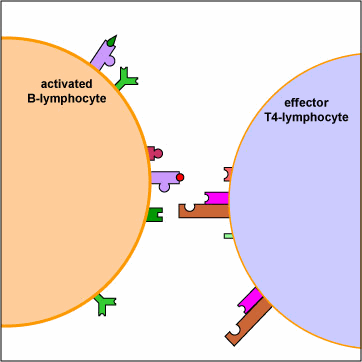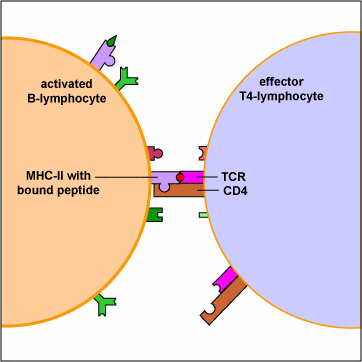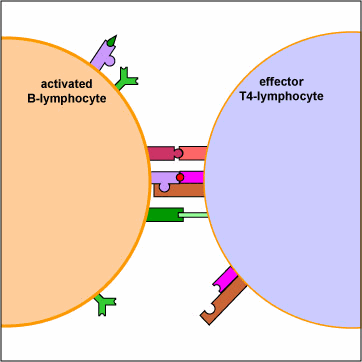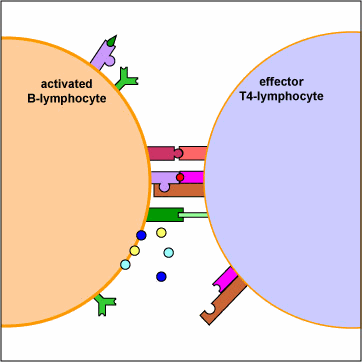
An effector T4-lymphocyte uses its TCR and CD4 molecule to bind to a complementary shaped MHC-II molecule with attached peptide epitope on an activated B-lymphocyte. This interaction, along with the binding of co-stimulatory molecules such as CD40 and B7 on the B-lymphocyte with their complementary ligands on the effector T4-lymphocyte triggers the T4-lymphocyte to produce cytokines that enable the activated B-lymphocyte to proliferate, differentiate into antibody-secreting B-lymphocytes and plasma cells, and switch classes of the antibodies being made.
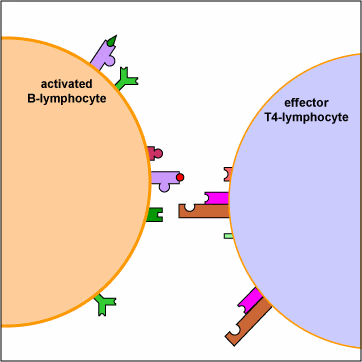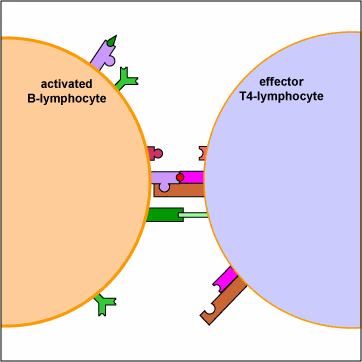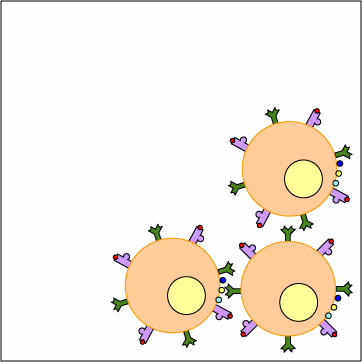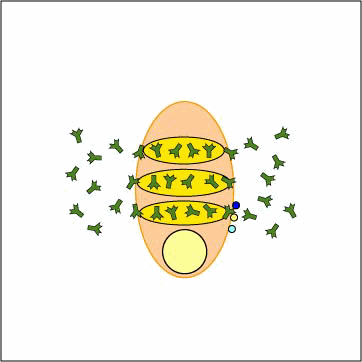
An effector T4-helper lymphocyte, by way of its TCR and CD4, binds to the MHC-II/epitope on the activated B-lymphocyte. This, along with co-stimulatory signals that result from the binding of costimulatory molecules such as CD40 and B7 on the B-lymphocyte with their corresponding ligands on the activated T4-lymphocyte enable the T4-helper cell to release cytokines such as Il-4, Il-5, Il-6, and Il-10. (The subset of T4-lymphocytes that normally interact with B-lymphocytes to promote antibody production are called Th2 lymphocytes.) These cytokines then bind to cytokine receptors on the activated B-lymphocyte triggering its proliferation into a large clone of identical B-lymphocytes. The clone of B-lymphocytes eventually differentiates into antibody-secreting B-lymphocytes and plasma cells. Some of the B-lymphocytes also differentiate into B-memory cells for heightened secondary response against that T-dependent antigen.
Cytokine release from T helper cell telling B cell to continue making Ab's that are specific to the immunogen.
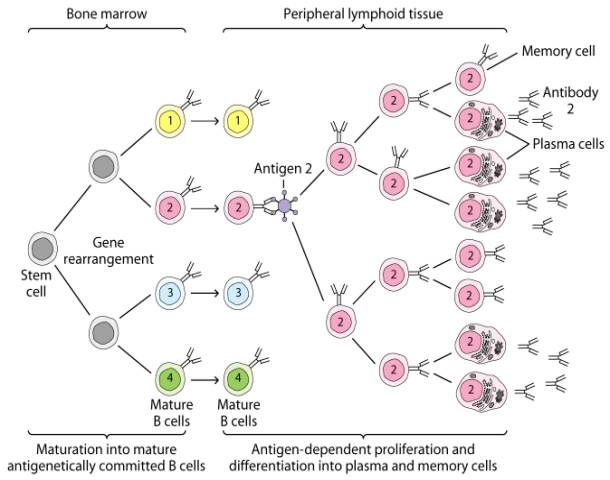
Q. What is the major difference between the B cell proliferation in this response compared to a low response?
MHC
· We know that transplants are rejected in a timely fashion (2 weeks)
· Blood transfusions are difficult
Why is this the case?
· Due to differences in the MHC
· MHC =
glycoproteins that are embedded in the plasma membrane of cells and act as
biochemical fingerprints unique to and individual.
Are highly polymorphic (genetically different)
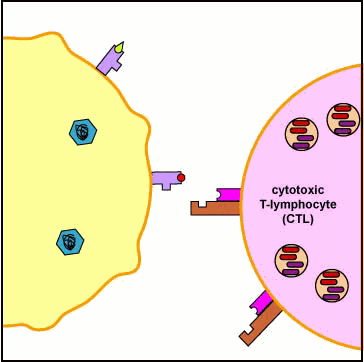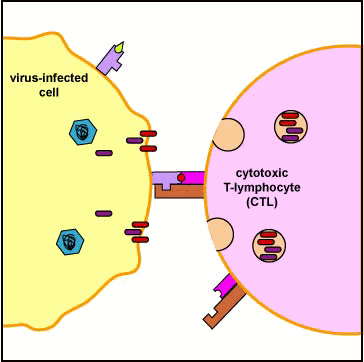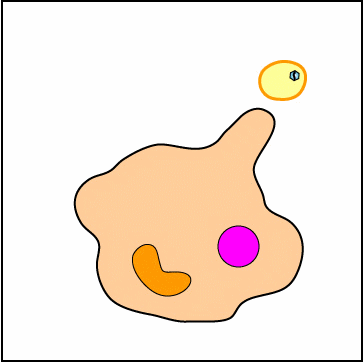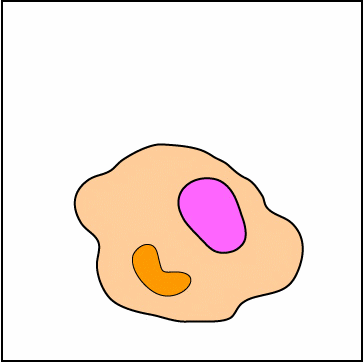
Binding of the CTL to the infected cell triggers the CTL to release pore-forming proteins called perforins and proteolytic enzymes called granzymes. Granzymes pass through the pores and activate the enzymes that lead to apoptosis, a programmed suicide of the infected cell. Apoptosis occurs when certain granzymes activate a group of protease enzymes called caspases that destroy the protein structural scaffolding of the cell, degrade the cell's nucleoprotein, and activate enzymes that degrade the cell's DNA. As a result, the infected cell breaks into membrane-bound fragments that are subsequently removed by phagocytes. If very large numbers of perforins are inserted into the plasma membrane of the infected cell, this can result in a weakening of the membrane and lead to cell lysis rather than apoptosis. An advantage to killing infected cells by apoptosis is that the cell's contents, including viable virus particles and mediators of inflammation, are not released as they are during cell lysis.